Kiwi researchers make a world-first breakthrough in the race for a Batten Disease cure
A campaign that started back in 2012 with the help of Flight of the Conchords and many leading New Zealand entertainers provided the crucial funding for South-island-based scientists to research gene therapies for Batten disease, a rare and fatal childhood condition.
Cure Kids is celebrating this year's Red Nose Day Appeal by marking a major breakthrough in the battle against Batten disease. A campaign that started back in 2012 with the help of Flight of the Conchords and many leading New Zealand entertainers provided the crucial funding for South-island-based scientists to research gene therapies for Batten disease, a rare and fatal childhood condition.
Fast forward ten years and these researchers from Lincoln University and the University of Otago have developed a world-first treatment for Batten disease, which has just been approved for trials in humans.
Batten disease is a rare inherited neurodegenerative disorder – children diagnosed with the cruel disease often only live to their teens. Their brain cells die away, and they suffer from symptoms similar to those of epilepsy, blindness, Alzheimer’s and Parkinson’s disease. There is no known cure for the disease.
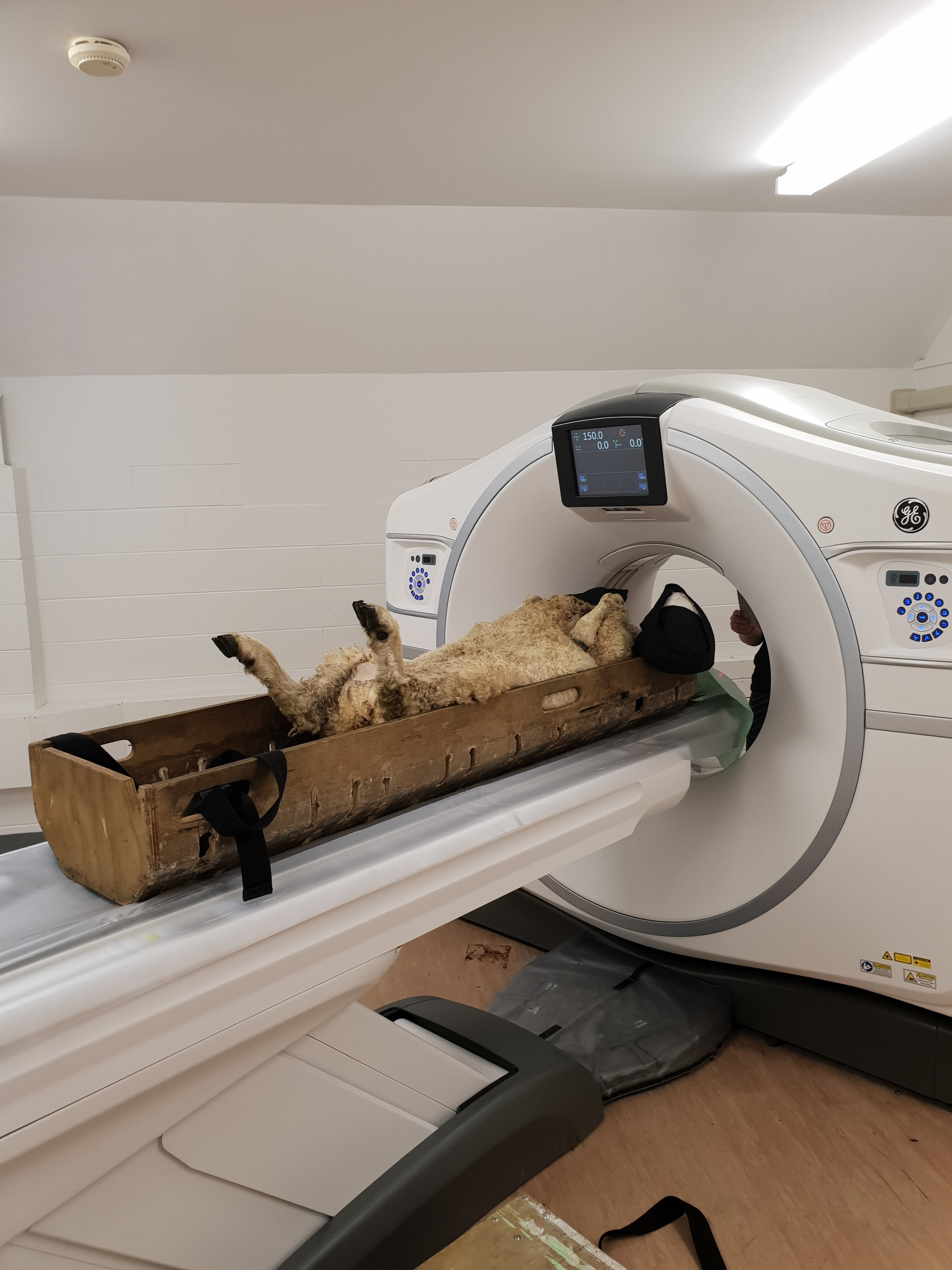
In 2021, the gene therapy for Batten disease developed by New Zealand researchers – and funded by Cure Kids (and others) – received US FDA approval for clinical trials. The New Zealand researchers are hopeful that results for humans will be similar to what they have seen in sheep. Sheep with Batten disease generally only live for two years, but the sheep given gene therapy are now living for more than five years.
“We can essentially halt the disease in sheep, so it’s not a complete cure. However, it may be that we are not seeing the cure playing out fully in sheep, but it could well happen in humans,” said Dr Nadia Mitchell, Research Group Lead at Lincoln University.
Because it is such a rare disease, this first clinical study is currently recruiting in the US, at the University of Rochester, New York. Funded by US-based biotech company, Neurogene, the trial is recruiting children between the ages of 3 and 8 years who have the rare CLN5 variant of Batten disease. The therapy involves a single injection of a specially created vector which carries a copy of the CLN5 gene to replace the faulty version in these children. The treatment will be delivered to the brain and eyes of each child, in the hope that the vector can penetrate the cells, carrying healthy copies of the CLN5 gene, and slow down or stop the effects of the disease.
The researchers will closely monitor children for five years after treatment to detect any potential safety issues, and assess any effects on children’s movement, speech, vision or brain function. Meanwhile, back in New Zealand, researchers at Lincoln University and the University of Otago will continue to investigate whether their breakthrough in gene therapy can be improved further, or adapted to treat different subtypes of the disease. They will work closely with the team at Neurogene throughout the human trials, providing advice and expertise.
Associate Professor Stephanie Hughes, who led the work at the University of Otago to develop the viral vector, is excited about the possibilities. “The FDA approval gives hope to thousands of families with Batten disease globally. We’re proud to see our science being taken to the next stage, and we are continuing to refine our methods for gene therapy and adapt it to help children with other variants of Batten disease.”
Cure Kids CEO Frances Benge is thrilled with the world-first breakthrough and the funding role that Cure Kids has played in enabling researchers to reach this milestone. “Every day, New Zealand researchers are working tirelessly to find treatments and cures for childhood illnesses and diseases, funded by the generosity of Kiwis. To have a world-leading breakthrough from some of the sharpest minds in the country, that could lead to better treatments for those suffering from Batten disease is a fantastic outcome. This research takes time, considerable investment and effort from all involved and we would like to thank all those who have been a part of this journey as well as those who have given so generously to Cure Kids.”
Donations to Cure Kids can be made at www.rednoseday.co.nz
About the Research
Decades of research in New Zealand’s South Island, in collaboration with top scientists around the world, preceded the development of the new gene therapy reaching human trials in the US.
Professor Bob Jolly from Massey University originally characterised sheep which have naturally-occurring forms of Batten disease, comparable to human variants CLN5 and CLN6.
At Lincoln University, a team including Professor David Palmer, Dr Nadia Mitchell, and Dr Samantha Murray have maintained two flocks of sheep, and worked with these sheep to study the disease pathology and test gene therapies for children. Their ongoing research concentrates of what impact the disease has on other organs in the body and whether their therapy may have other benefits to these cells in CLN5 and CLN6 variants.
At the University of Otago, Associate Professor Stephanie Hughes has developed viral vectors which can deliver the gene therapy to the correct cells in the best way to ensure that it can replace normal cellular function. With her team, including Dr Indranil Basak, Dr Luci Schweitzer, and Hollie Wicky, she been working to understand the molecular and cellular processes by which mutations in the CLN genes cause the symptoms of Batten disease. They’ve developed models in human cells and mice to optimise gene therapy strategies in Batten disease.
Cure Kids is keen to raise enough funds to continue to support this world-leading research at both the University of Otago and Lincoln University.
Available for media interviews
- Dr Nadia Mitchell – Lincoln University
- Associate Professor Stephanie Hughes – University of Otago
- Family of Katie Archer – who died of Batten disease in 2018, age 10 (Katie’s mother, Lisa).
- Frances Benge, Cure Kids CEO
Media contact
Ashleigh Gilchrist
ashleigh@anthem.co.nz
021 236 8324